electron configuration for ni2+|Electron Configuration for Nickel (Ni and Ni2+, Ni3+ ions) : iloilo By “building up” from hydrogen, this table can be used to determine the electron configuration for any atom on the periodic table. We will now construct the ground-state electron configuration and orbital diagram for a selection of . Word to numbers examples 89.5 trillion 70.1 trillion 8.5 trillion 81.4 trillion 57.5 k 43.6k 10.2 trillion 12 trillion 1.4k 61.7 billion 99.2k 22.5 k 19.3 k 18.4 million References:
electron configuration for ni2+,To write the configuration for the Nickel ions, first we need to write the electron configuration for just Nickel (Ni). We first need to find the number of electrons for the Ni atom (there. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator.
In this video we’ll use the Periodic table and a few simple rules to find the number of protons and electrons for neutral Nickel (Ni) and the Nickel ions (Ni.By “building up” from hydrogen, this table can be used to determine the electron configuration for any atom on the periodic table. We will now construct the ground-state electron configuration and orbital diagram for a selection of .
First, write the electron configuration for the neutral atoms: Zn: [Ar]3d 10 4s 2; Cr: [Ar]3d 5 4s 1; Next, remove electrons from the highest energy orbital. For the transition metals, electrons .
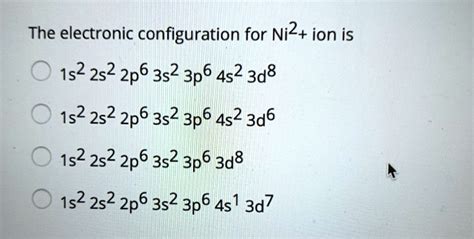
Determine the electron configuration of ions. Justify the observed charge of ions to their electronic configuration. Define paramagnetism and diamagnetism. Justify the anomalies of . Ni2+ electron configuration is 1s22s22p63s23p63d84s2. The atomic radious of Ni2+70 pm. Ni2+ having fcc (face centre cubic ) crystal structure. From the electronic . Explanation: Electronic configuration of Nickel ( 28Ni) is. 1s2 2s2 2p6 3s2 3p6 4s2 3d8. After removal of two electrons from outermost shell the electron configuration of Ni2+ is. .
electron configuration for ni2+ Electron Configuration for Nickel (Ni and Ni2+, Ni3+ ions)Introduction to the chemistry of nickel. 2. Data tables for the chemistry of nickel. 3. Uses of nickel. 4. Electron configuration, oxidation states and electrode potentials. 5. Nickel (II) chemistry .The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells .
However the $$4s$$ electrons are further from the nucleus so losing the $$2\ 4s$$ electrons leaves only the third shell electrons making the atoms more stable than losing the $$3d$$ electrons.electron configuration for ni2+NOTE: Chromium is an exception to the rules for writing electron configurations! Video: Cr, Cr 2+, and Cr 3+ Electron Configuration Notation In writing the electron configuration for Chromium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Chromium go in the 2s orbital. The electron configuration for Ni is [Ar]3d8 4s2. Since it says Ni2+, the 2 plus charge means that this is a Ni cation that has lost 2 electrons. These 2 electrons are lost from the electron orbital with the highest energy, which in this case is the 4s orbital.Ni2+: From Table 2.2, the electron configuration for an atom of nickel is 1s22s22p63s23p63d84s2. In order to become an ion with a plus two charge, it must lose two electrons—in this case the two 4s. Thus, the electron configuration for a Ni2+ ion is 1s22s22p63s23p63d8.
Electronic configuration: An element's electron configuration describes the distribution of electrons in its atomic orbitals. It tells us the number of lone pairs of electrons, magnetic properties, etc. Ni has an atomic number = 28 with electronic configuration = 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 8 + 2 charge indicates it has lost two .The same rule will apply to transition metals when forming ions. You should note that the ns electrons are always lost before the (n-1)d when forming cations for transition metals.For example, the electron configuration for Zn: [Ar]4s 2 3d 10 . the electron configuration for Zn +2: [Ar]3d 10 . The transition metals still do not end up being isoelectronic with a noble gas, .
Electron Configuration for Nickel (Ni and Ni2+, Ni3+ ions)Question: Which, if any, is the ground-state electron configuration for Ni2+? O [Ar]3d or 1s22s22p63523p63d6 O [Ar]3d? or 1s22s22p63523p63d? [Ar]3d8 or 1s22s22p63s23p63d8 [Ar]3dº or 1s22s22p63s23p63dº None of the above . Show transcribed image text. There are 2 steps to solve this one.

The AFM state is close to a half-filled configuration for every Ni site, while the CO state has a configuration in which Ni2 site has almost zero occupancy and Ni1/Ni3 site is half-filled. The Order of Filling Orbitals. The aufbau principle explains how electrons fill low energy orbitals (closer to the nucleus) before they fill higher energy ones. Where there is a choice between orbitals of equal energy, they fill the orbitals singly as far as possible (Hunds rules).The diagram (not to scale) summarizes the energies of the orbitals up to the 4p level.

Question: Write the electron configuration for Ni2+. Write the electron configuration for Ni2+. Here’s the best way to solve it. 100 % .
Ni2+ has the condensed electron configuration, Ni2+: [Ar] 3d8. Ni3+ has the condensed electron configuration, Ni3+: [Ar] 3d7. Here we see that Nickel loses its first two electrons from the s-orbital. Next, the electrons leave the d-orbital. Electron Configurations of the Lanthanides. The Lanthanides are in the sixth period of the periodic table .Electronic states of the NiCu molecule determined byab initio Hartree-Fock and configuration interaction methods. Theoretica Chimica Acta 1980 , 54 (2) , 113-122.5. NICKEL (II) CHEMISTRY and complexes. Electron configuration of Ni 2+ is [Ar]3d 8. In aqueous solution nickel forms the green stable hexaaquanickel(II) ion, [Ni(H 2 O) 6] 2+ (aq) from eg nickel(II) chloride solution NiCl 2(aq) or nickel(II) sulfate NiSO 4(aq), both of which are suitable for laboratory experiments for investigating the aqueous chemistry of the nickel(II) ion.Write the electron configuration of the Ni2+ ion. ii) What are the possible values of the total spin quantum numbers S and Ms for this ion There are 3 steps to solve this one.Question: Select the correct complete electron configuration for the nickel (II) ion, Ni2+. [Ar] 3d8 4s2 [Ar] 4s2 3d8 [Ar] 3d8 [Ar] 3d10 4s2 Select the correct complete electron configuration for the nickel (II) ion, Ni 2+ .However the $$4s$$ electrons are further from the nucleus so losing the $$2\ 4s$$ electrons leaves only the third shell electrons making the atoms more stable than losing the $$3d$$ electrons.
In order to write the Copper electron configuration we first need to know the number of electrons for the Cu atom (there are 29 electrons). Once we have the configuration for Cu, the ions are simple. When we write the configuration we'll put all 29 electrons in orbitals around the nucleus of the Copper atom. NOTE: Copper is an exception to the .
electron configuration for ni2+|Electron Configuration for Nickel (Ni and Ni2+, Ni3+ ions)
PH0 · What is the electron configuration of #Ni^(2+)#?
PH1 · Nickel transition metal Chemistry nickel(II) Ni2+ complex ions
PH2 · Ni2+ Electron Configuration(Explained for Beginners)
PH3 · Ni2+ Electron Configuration(Explained for Beginners)
PH4 · How to find Protons & Electrons for the Ni, Ni2+, and Ni3+
PH5 · How many unpaired electrons are there in Ni2+? Q&A
PH6 · Electron Configuration for Nickel (Ni and Ni2+, Ni3+ ions)
PH7 · Electron Configuration for Ni, Ni2+, and Ni3+ (Nickel and
PH8 · Electron Configuration for Ni, Ni2+, and Ni3+ (Nickel and
PH9 · Electron Configuration Calculator
PH10 · 9.6: Electron Configurations of Ions
PH11 · 7.4: Electron Configurations of Ions
PH12 · 3.1: Electron Configurations